

Thank you for Subscribing to Medical Care Review Weekly Brief

 To address these challenges, EU4Health and Cancer Mission (see Box 1) have funded the projects PCM4EU and PRIME-ROSE . PCM4EU is a consortium of 17 partners from 15 countries that focuses on the deployment of novel precision cancer medicine diagnostic tools, whereas PRIME-ROSE is treatmentoriented and consists of 24 partners, from altogether 18 European countries focusing on accelerating precision medicine implementation. The consortiums are coordinated by Professor Hans Gelderblom at the Leiden University Medical Center and Professor Kjetil Tasken at Oslo University Hospital, respectively. Together, this EU-wide precision cancer medicine deployment addresses key scientific questions, aiming to improve access to new and effective cancer treatments.
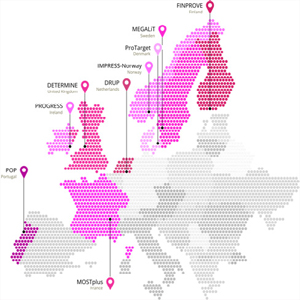
PCM4EU and PRIME-ROSE build on a family of clinicianinitiated precision cancer medicine clinical trials called DRUP-like clinical trials, which have been particularly successful (see Figure 1). Most of the trials have been running for more than two years and have access to a portfolio of cancer drugs with 10-30 drugs and a proven record of patient inclusion. The trials share the pragmatic clinical trial design of the original Dutch DRUP trial that opened already in 2016, with broad inclusion criteria and a limited set of endpoints. The DRUP-like clinical trials offer new treatment alternatives for patients with advanced cancer with an actionable target who are progressing on standard treatment, but with sufficient quality of health to anticipate benefit from another line of treatment. The trials investigate approved drugs on new indications based on the patient’s molecular profile and on tumor type. There are currently seven DRUP-like trials running in Europe, and more is expected the coming years.
PCM4EU was initiated in January 2023, is evaluating current standards and will define best practice, describe state-of-the-art molecular diagnostics, and set out the road to implementation. An important part of the project is education, and several podcasts and webinars have already been prepared and uploaded on the PCM4EU website (pcm4eu.eu). Moreover, through harmonization and collaboration, the consortium will facilitate mechanisms for interpreting molecular and clinical data, thereby anticipating the benefits of the upcoming European Health Data Space.
To address these challenges, EU4Health and Cancer Mission (see Box 1) have funded the projects PCM4EU and PRIME-ROSE . PCM4EU is a consortium of 17 partners from 15 countries that focuses on the deployment of novel precision cancer medicine diagnostic tools, whereas PRIME-ROSE is treatmentoriented and consists of 24 partners, from altogether 18 European countries focusing on accelerating precision medicine implementation. The consortiums are coordinated by Professor Hans Gelderblom at the Leiden University Medical Center and Professor Kjetil Tasken at Oslo University Hospital, respectively. Together, this EU-wide precision cancer medicine deployment addresses key scientific questions, aiming to improve access to new and effective cancer treatments.
PCM4EU and PRIME-ROSE build on a family of clinicianinitiated precision cancer medicine clinical trials called DRUP-like clinical trials, which have been particularly successful (see Figure 1). Most of the trials have been running for more than two years and have access to a portfolio of cancer drugs with 10-30 drugs and a proven record of patient inclusion. The trials share the pragmatic clinical trial design of the original Dutch DRUP trial that opened already in 2016, with broad inclusion criteria and a limited set of endpoints. The DRUP-like clinical trials offer new treatment alternatives for patients with advanced cancer with an actionable target who are progressing on standard treatment, but with sufficient quality of health to anticipate benefit from another line of treatment. The trials investigate approved drugs on new indications based on the patient’s molecular profile and on tumor type. There are currently seven DRUP-like trials running in Europe, and more is expected the coming years.
PCM4EU was initiated in January 2023, is evaluating current standards and will define best practice, describe state-of-the-art molecular diagnostics, and set out the road to implementation. An important part of the project is education, and several podcasts and webinars have already been prepared and uploaded on the PCM4EU website (pcm4eu.eu). Moreover, through harmonization and collaboration, the consortium will facilitate mechanisms for interpreting molecular and clinical data, thereby anticipating the benefits of the upcoming European Health Data Space.
 PRIME-ROSE was launched in July 2023. The project takes advantage of the adaptive and pragmatic DRUP-like clinical trials to create a platform to answer key questions regarding clinical effectiveness, provide health-economic evaluations, and contribute to scientific progress across cancers. In the project, data will be aggregated from all the trials to provide knowledge regarding efficacy and safety. This will create a synthetic multi-national trial while keeping the national aspects of each trial, which is essential for implementation. The cross-border collaboration is especially relevant for rare tumor types and molecular profiles, which are enriched in DRUP-like clinical trials. Cohorts of patients with rare mutations and/ or tumor types can, through this cross-trial collaboration, be filled simultaneously in multiple countries to obtain answers more efficiently and build the knowledge base faster. This provides a route for pharma partners to join all the trials, concomitantly increasing effectiveness in finding patients with specific biomarkers. Furthermore, PRIME-ROSE will provide randomized synthetic control arms for evaluating clinical efficacy and cost-effectiveness and strategies for implementation adapted to the different countries. To ensure successful implementation, the consortium will work together with regulators, policymakers, payers, healthcare providers, and patient advocacy groups to build a European ecosystem for the implementation of precision cancer medicine.
Through European collaboration, PCM4EU and PRIMEROSE can contribute significantly within the field of precision cancer medicine to efficiently match drugs with patients and evaluate treatment efficacy. This can be used to evaluate the treatment efficacy of new drugs, provide a systematic approach for reimbursement, and improve patient benefit by exploring drugs in all tumor types.
The projects will contribute to a sustainable and equitable implementation of precision cancer medicine in Europe, improving overall survival and quality of life for cancer patients.
PRIME-ROSE was launched in July 2023. The project takes advantage of the adaptive and pragmatic DRUP-like clinical trials to create a platform to answer key questions regarding clinical effectiveness, provide health-economic evaluations, and contribute to scientific progress across cancers. In the project, data will be aggregated from all the trials to provide knowledge regarding efficacy and safety. This will create a synthetic multi-national trial while keeping the national aspects of each trial, which is essential for implementation. The cross-border collaboration is especially relevant for rare tumor types and molecular profiles, which are enriched in DRUP-like clinical trials. Cohorts of patients with rare mutations and/ or tumor types can, through this cross-trial collaboration, be filled simultaneously in multiple countries to obtain answers more efficiently and build the knowledge base faster. This provides a route for pharma partners to join all the trials, concomitantly increasing effectiveness in finding patients with specific biomarkers. Furthermore, PRIME-ROSE will provide randomized synthetic control arms for evaluating clinical efficacy and cost-effectiveness and strategies for implementation adapted to the different countries. To ensure successful implementation, the consortium will work together with regulators, policymakers, payers, healthcare providers, and patient advocacy groups to build a European ecosystem for the implementation of precision cancer medicine.
Through European collaboration, PCM4EU and PRIMEROSE can contribute significantly within the field of precision cancer medicine to efficiently match drugs with patients and evaluate treatment efficacy. This can be used to evaluate the treatment efficacy of new drugs, provide a systematic approach for reimbursement, and improve patient benefit by exploring drugs in all tumor types.
The projects will contribute to a sustainable and equitable implementation of precision cancer medicine in Europe, improving overall survival and quality of life for cancer patients. 